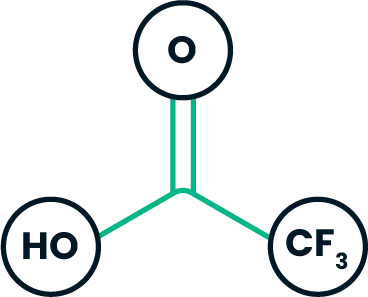
Trifluoroacetic acid (TFA) reacts with active hydrogen (HZ, such as hydroxy and amino groups):
CF3COOH + HZ —–> CF3COZ + HOH
When using TFA, water is the coproduct, which works well in aqueous systems or systems tolerant of water.
The reactions of TFA are centered around the carbonyl group. The trifluoromethyl group, on the other hand, is one of the most stable organic structures. It is inert toward practically all oxidizing, reducing and hydrolyzing conditions and in vivo metabolism. The carbonyl group provides a way to attach the trifluoromethyl group to other molecules.
The usual carboxylic acid derivatives such as esters and amides can be made readily. They differ from the corresponding acetic acid derivatives in that the esters hydrolyze more readily and the amides of primary and secondary amines are sufficiently acidic to dissolve in 5% aqueous alkali.
TFA adds to olefins and acetylenes. The saturated ester from the olefin addition can be hydrolyzed readily to the corresponding alcohol. In fact, TFA can be used to promote hydration of olefins which are too sensitive to be hydrated in the usual sulfuric acid procedure.
TFA is used as an esterification and transesterification promoter. In the presence of another carboxylic acid, that acid will be preferentially esterified. The mixed anhydride can be made from the other acid anhydride and TFA.
TFA can be used to promote the acylation of aromatic and unsaturated compounds to form the corresponding ketones and a variety of acyl-containing compounds. TFA can be used to protect active amino groups temporarily. Esters of amino acids protected this way have sufficient volatility to be distilled or analyzed by GLC. Removal of the TFA is easily accomplished without changing chirality or other active groups in the molecule.
In some automated peptide reactions, TFA is added to deprotect amino groups sequentially as the molecule is being built. For this use, the solvent characteristics, acidity and volatility of TFA play a role. Our highly purified, lowresidue BioGrade™ TFA helps assure purity in the final protein.
Making trifluoroacyl derivatives permits the use of 19F NMR. Analysis of the chemical shifts can aid in determining the structure of the original molecule. TFA can be mixed with other acids, or Lewis acids, to generate modified catalysts useful in olefin/alkane reactions in petroleum refining steps. These catalysts reduce byproduct sludges and increase yields when compared to the more conventional catalysts.
Peroxytrifluoroactetic acid is readily made from TFA and hydrogen peroxide. The peroxy acid is an oxidizer of amines, olefins, ketones, oximes and aromatics. The combination of the peroxy acid and boron trifluoride is an excellent source of positive hydroxyl groups for the hydroxylation of aromatic compounds.
The peroxy acid will dissolve metals not normally dissolved by mineral acids (Ag, Bi, Cu, Hg, In, Pb, Tl, etc.). This is useful for analytical purposes. Another analytical use for TFA is as the solvent during oxidations of metal ions by chromic acid, permanganate and chlorine. TFA is both inert and a good solvent in these cases.
TFA is miscible with most organic solvents and it will dissolve limited quantities of lower alkanes. Fluorinated solvents, even perfluorocarbons, are completely miscible with TFA. Polyamides and polyesters are soluble and the solutions can be used for analytical purposes or to perform reactions on the polymers. The solutions may also be used to apply coatings of the polymer or to spin fibers at low temperatures.