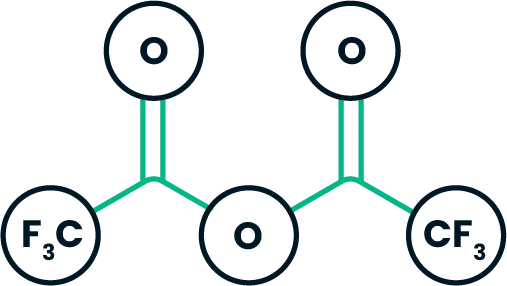
Trifluoroacetic anhydride provides a convenient way to introduce a trifluoromethyl group into an organic compound. It is used in the production of agricultural and pharmaceutical molecules.
It is also used heavily in chromatography. TFAA is the most volatile and reactive of the anhydrides and it reacts with alcohols, amines, and phenols.
Applications
• Organic synthesis
• NMR solvent
• Strong acidity
• Trifluoromethyl group
• Gas chromatography
• Liquid chromatography
Suitable for
• Use as catalyst for a wide range of reactions including esterifications, condensations, and oxidations
• Esterification and transesterification promoter
• Promotes condensation reactions involving acyl groups and aromatic or unsaturated compounds
• Solvent for chromatography