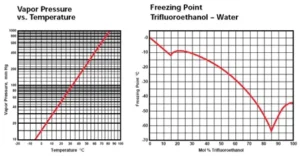
Trifluoroethanol, CF3CH2OH, was first prepared in 1933. This colorless liquid with an ethanol-like odor has a number of unique properties. The three fluorines give the alcohol a high ionization constant and very strong hydrogen bonding capability, which make it a very unusual solvent. The fluorines also make the molecule very stable at high temperatures. These properties have put trifluoroethanol (TFE) to use in some special applications in addition to its use in pharmaceutical and agricultural chemical syntheses.
In 1960, Halocarbon Life Sciences was the world’s first commercial producer of TFE. Today, with our state-of-the-art plant in North Augusta, South Carolina, we continue in the forefront supplying this important and interesting compound.
Key Features
• High ionization constant
• Very strong hydrogen bonding capability
• Stable at high temperatures
• Dissolves nylons at room temperature
Suitable for
• Common metals, including carbon steel
Resistant to
• Common metals, including carbon steel