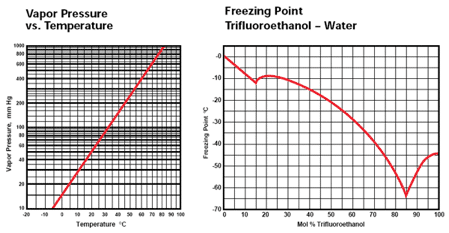
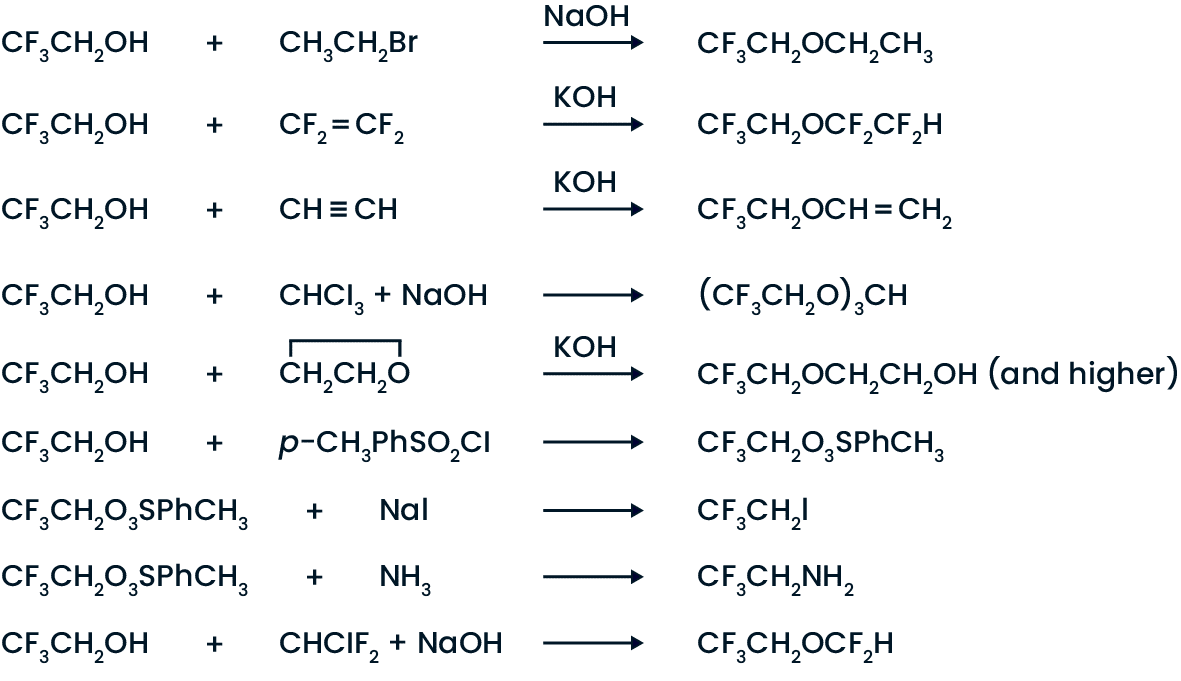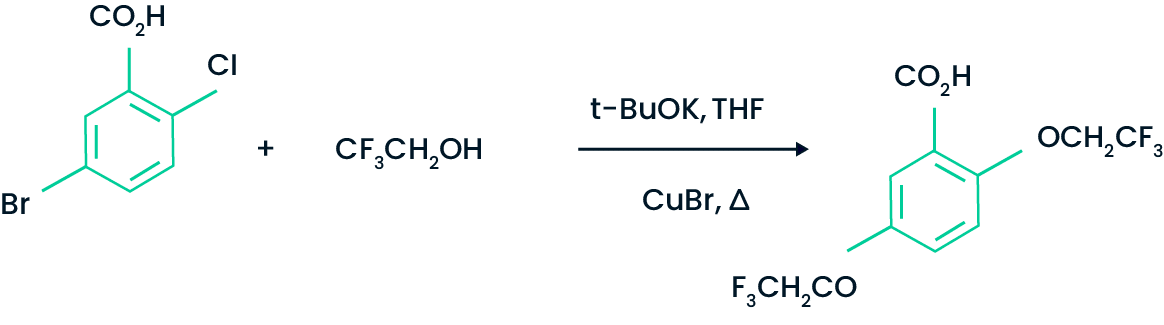The presence of the three fluorines in the molecule make TFE resemble the phenols more than ordinary alcohols, but it does undergo reactions typical of both. Some typical reactions of TFE are:
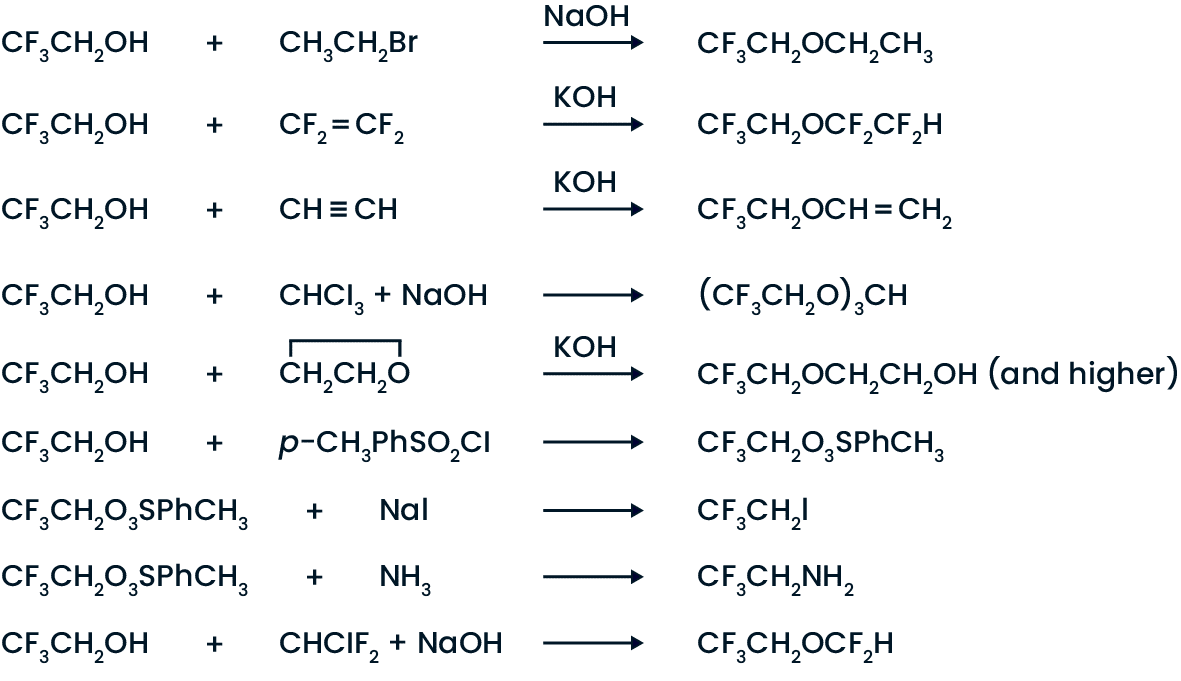
An example from the recent patent literature is shown below.
Process for preparing a trifluoroethoxy substituted benzoic acid
US 6849762, 2005, Merck Patent Gmbh
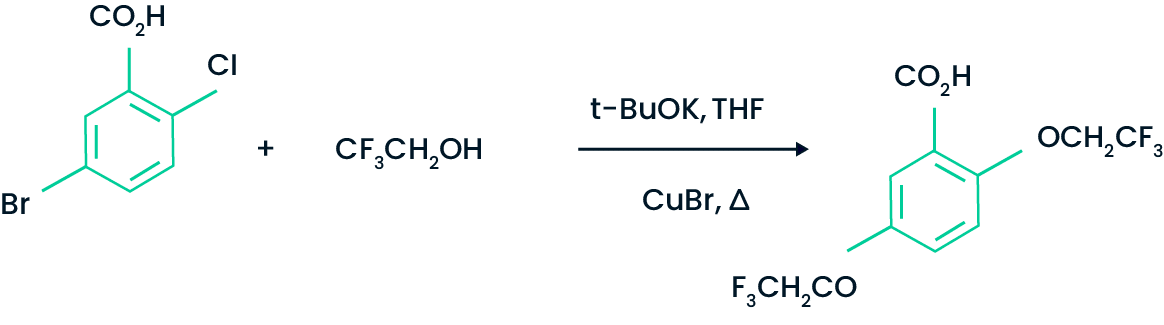
Solubilities
TFE is completely miscible with water, alcohols, ketones, esters, ethers and other oxygen containing solvents. TFE is miscible with lower aliphatic and aromatic hydrocarbons, but not with higher ones. Carbon tetrachloride is only slightly miscible with TFE but chloroform and methyl chloroform are completely miscible.
An unusual property of TFE is its ability to dissolve nylons at room temperature. Unlike nylon solutions in strong acids, the nylons can be recovered unchanged from these solutions. Concentrations of 3 to 10% may be made from common nylons at room temperature and metastable solutions can be made from more concentrated hot solutions upon cooling. These supersaturated solutions may remain clear for hours or even days at room temperature, serving as a convenient source of fluid nylon.
Nylon is much less soluble in TFE containing hydrogen bonding liquids such as water and other alcohols. If done carefully, the addition of water to a nylon solution can be used for the stepwise determination of the molecular weight distribution of the polymer because the higher molecular weight polymers precipitate at lower water concentrations. Polymer solubility in TFE extends to oxygen containing amorphous polymers. Polymethacrylates, polyvinylacetate, cellulose acetate and many others are extremely soluble. However, the more crystalline polymers such as the polyaldehydes, polycarbonates and aromatic polyesters are insoluble. The aliphatic polymers are also insoluble. Common gases have solubility in TFE similar to their solubility in water. Nitrogen is soluble to the extent of 0.06 mL of gas per mL of liquid at 27 °C. For oxygen the value is 0.13 and for CO2 the value is 1.8. Inorganic salts are slightly soluble in TFE containing 0.2% water but are two to three times more soluble in TFE containing 5% water. The combination of TFE and water is convenient for organic ionic reactions and conductometric titrations.