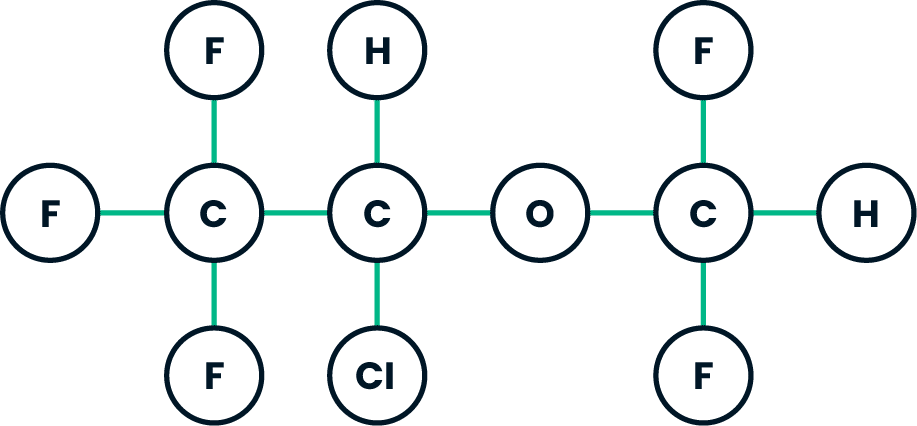
General: As with any potent general anesthetic isoflurane should only be administered in an adequately equipped anesthetizing environment by those who are familiar with the pharmacology of the drug and qualified by training and experience to manage the anesthetized patient. Regardless of the anesthetics employed, maintenance of normal hemodynamics is important to the avoidance of myocardial ischemia in patients with coronary artery disease. Isoflurane, like some other inhalational anesthetics, can react with desiccated carbon dioxide (CO2) absorbents to produce carbon monoxide, which may result in elevated levels of carboxyhemoglobin in some patients. Case reports suggest that barium hydroxide lime and soda lime become desiccated when fresh gases are passed through the CO2 absorber canister at high flow rates over many hours or days. When a clinician suspects that CO2 absorbent may be desiccated, it should be replaced before the administration of isoflurane. As with other halogenated anesthetic agents, isoflurane may cause sensitivity hepatitis in patients who have been sensitized by previous exposure to halogenated anesthetics (see CONTRAINDICATIONS).
Information to Patients: Isoflurane, as well as other general anesthetics, may cause a slight decrease in intellectual function for 2 or 3 days following anesthesia. As with other anesthetics, small changes in moods and symptoms may persist for up to 6 days after administration.
Laboratory Tests: Transient increases in BSP retention, blood glucose and serum creatinine with decrease in BUN, serum cholesterol and alkaline phosphatase have been observed.
Drug Interactions: Isoflurane potentiates the muscle relaxant effect of all muscle relaxants, most notably nondepolarizing muscle relaxants, and MAC (minimum alveolar concentration) is reduced by concomitant administration of N2O. See CLINICAL PHARMACOLOGY.
Pregnancy Category C: Isoflurane has been shown to have a possible anesthetic-related fetotoxic effect in mice when given doses 6 times the human dose. There are no adequate and well-controlled studies in pregnant women. Isoflurane should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: It is not known whether this drug is excreted in human milk.
Malignant Hyperthermia: In susceptible individuals, isoflurane anesthesia may trigger a skeletal muscle hypermetabolic state leading to high oxygen demand and the clinical syndrome known as malignant hyperthermia.